Vanessa Biotech registers VR-AD-1005, a novel antidiarrheal investigational drug, for Phase II clinical trial with the Pan African Clinical Trials Registry for treatment of severe diarrhea of cholera.
Vanessa Biotech, the Hamden, Conn. based global biomedical company dedicated to making an impact on healthcare markets, has registered VR-AD-1005, a novel antidiarrheal investigational drug, with the Pan African Clinical Trials Registry (PACTR) system, as of June 21, 2021. The drug will be studied for the innovative treatment of severe diarrhea of cholera, heralding a pipeline of drugs developed in hopes of neutralizing the lethal diarrhea of cholera as well as rotaviral diarrhea and traveler’s diarrhea. VR-AD-1005 is intended to work by reducing the volume of liquid stool produced by the intestine when suffering from the diarrheal pathogen. The drug works within the intestine without affecting its motility and does not induce constipation. If successful—unlike other antidiarrheal medications—VR-AD-1005 will be the first symptomatic medication to treat severe secretory diarrhea. “Our unique approach to treatment of diarrhea concentrates on solving the problem of continuous removal of antidiarrheal medications with enormous volume of stool in patients suffering from acute watery diarrhea. This problem is known as convective washout, and it is recognized as the major factor limiting efficacy of oral antidiarrheal medicines. Vanessa Biotech is the first company that has developed a solution for it,” according to Dr. Dmitry Kravtsov, Vice President of Research and Development at Vanessa Biotech.
* These claims are based on preclinical studies and observed results in clinical trials to date. As clinical trials have not been completed, there can be no definitive proof these claims will ultimately be proven to be factual.
About Vanessa Biotech
Vanessa Biotech (VB) is a biomedical company founded on the desire to “give hope where none existed” by making an impact on healthcare markets often overlooked. Its continually expanding pipeline of innovative pharmaceuticals, medical devices and consumer and public health products reflects the company’s inventive nature and commitment to delivering solutions that increase access to quality care. The company’s flagship product, Shylicine™, is the first-ever drug developed to treat the rare and lethal microvillus inclusion disease. Vanessa Biotech, a University of Connecticut Technology Incubation Program participant, has global offices in Hungary, Spain and Germany in addition to its Hamden, Conn. corporate campus.
Contact Information
Vanessa Biotech
P.O. Box 185755
Hamden, Conn. 06518 USA
+1 203 836 8424
Contact Us
Vanessa Research (VR)—a New Haven-based biomedical company committed to improving patient quality of life by developing innovative products and solutions in areas of unmet medical needs— will support and participate in Rare Disease Day 2020 for the second year. Dr. Dmitry Kravtsov, VP of Research and Development at VR, will be serving as a Rare Disease Ambassador at Quinnipiac University’s North Haven Campus on February 28th, 2020 at 1:00PM. Dr. Kravtsov will be available to discuss the impact of his work with Microvillus Inclusion Disease (MVID)—a rare, pediatric, gastrointestinal disorder—with other attendees.
25-30 million Americans are impacted by rare diseases—rare being defined as any disease that affects less than 200,000 people in the U.S. Each year on the last day of February, a month known for its “rare” number of days, the rare disease community coalesces to raise awareness for the one in ten Americans suffering from rare diseases.
The global theme for Rare Disease Day 2020 is “Equity.” This theme embodies working together worldwide “towards more equitable access to diagnosis, treatment, care and social opportunity”1 for those living with a rare disease.
Commenting on his upcoming role, Dr. Kravtsov stated: “The rarity of MVID makes medical research on the condition difficult for caregivers and doctors alike. As a Rare Disease Ambassador, I hope to raise awareness on MVID and expand public knowledge of rare diseases—of which more than half of the affected population are children.”
VR has been dedicated to raising awareness about MVID since its inception, recently by writing a white paper in 2019 that is the first and only comprehensive case analysis that summarizes data trends across all known literature of MVID, and sharing our knowledge of MVID in an interview with Health Professional Radio. VR has also made significant strides within the rare disease community including gaining approval to conduct phase II clinical trials of Shylicine®, the first-ever investigational treatment developed for MVID. Participating in Rare Disease Day 2020 provides another opportunity for VR to further their initiative of shedding light on MVID and their ongoing mission to “give hope where none existed.”
About Vanessa Research
Vanessa Research (VR) is a biomedical company founded on the desire to “give hope where none existed” by making an impact on healthcare markets that are often overlooked. VR’s continually expanding pipeline of innovative pharmaceuticals, medical devices, and consumer and public health products reflects the company’s inventive nature and commitment to delivering solutions that increase access to quality care. The company’s flagship product, Shylicine®, is the first-ever drug developed to treat the rare, lethal microvillus inclusion disease.
VR is based in New Haven, Connecticut, with offices in Budapest, Hungary; Munich, Germany; and Navarre, Spain.
Contact Information
Christina McGrath
Director of Marketing and Communications
Vanessa Research
925 Sherman Avenue,
Hamden, CT 06514 USA
christina.mcgrath@vanessaresearch.com
www.vanessaresearch.com
To attend Rare Disease Day at Quinnipiac please register and visit: QU Rare Disease Day 2020
Event Location and Time:
Frank H. Netter MD School of Medicine 370 Bassett Road, North Haven, CT 06473 Room MNH 101 February 28th at 1:00PM
1 Get Inspired. (n.d.). Retrieved from https://rarediseases.org/rare-disease-day/get-inspired/
Vanessa Research shares their organizational growth and strategic objectives with healthcare professionals worldwide
BUDAPEST, January 16, 2020 — Vanessa Research, (VR) — a biomedical company transforming the healthcare landscape by developing and commercializing innovative products in areas of unmet medical needs— participated in an interview with Pharma Boardroom, the premier website for C-Level executives, consultants, regulators and vendors working in Healthcare & the Life Sciences globally. László Dinca, Managing Director of Vanessa Research Hungary, joined Pharma Boardroom to share his insights on the company’s rare disease portfolio and their entrance into the pharmaceutical contract manufacturing landscape. The interview is available at pharmaboardroom.com/interviews/laszlo-dinca-managing-director-vanessa-research-hungary/.
During the interview, Dinca addressed the strategic intentions behind the recent acquisition of HG Biotech (f/k/a/ Hungaro-Gal Pharmaceutical Manufacturing), the significance of Hungary for VR, and the future direction of the company. He highlighted how the acquisition of HG Biotech gave VR manufacturing capabilities that allows them to control and ensure the supply and the quality of their medicine as well as facilitate VR’s access to the European market once Shylicine™, a cocktail drug candidate for treating Microvillus Inclusion Disease (MVID), is authorized.
Pharma Boardroom interviews with top pharma companies worldwide and releases a global Health & Life Sciences Review annually, an opportunity to showcase the significant advancement of health and wellness underway throughout the nation. The 2020 edition of the Hungary Healthcare & Life Sciences Review will include VR as a key player in the Hungarian pharmaceutical landscape.
In response to a question regarding the company’s five-year agenda, Dinca stated: “The main mission is to produce drugs for MVID and cholera. Furthermore, we aim to solidify the strong foundations of the contract manufacturing operations at HG Biotech, and to build upon them. The idea is to bring these drugs to patients as quickly as possible and we believe that in-house manufacturing is the best way forward for us. …VR aims to retain and strengthen relationships with HG Biotech’s current clients while simultaneously working to build new partnerships.”
With their newly formed division: pharmaceutical manufacturing, VR is one step closer to achieving their long-term vision of bringing medicines to patients around the world, and serving overlooked healthcare markets. VR plans to continue creating an added value for their patients, the healthcare industry, and the Hungarian business ecosystem, fulfilling the company’s mission to “give hope where none existed.’’
About Vanessa Research
Vanessa Research (VR) is a biomedical company founded on the desire to “give hope where none existed” by making an impact on healthcare markets that are often overlooked. VR’s continually-expanding pipeline of innovative pharmaceuticals, medical devices, and consumer and public health products reflects the company’s inventive nature and commitment to delivering solutions that increase access to quality care. The company’s flagship product, Shylicine™, is the first-ever drug developed to treat the rare, lethal microvillus inclusion disease.
VR is based in Hamden, Connecticut, with offices in Budapest, Hungary; Munich, Germany; and Navarre, Spain.
Contact Information
Christina McGrath
Director of Marketing and Communications
Vanessa Research
925 Sherman Avenue,
Hamden, CT, 06514 USA
christina.mcgrath@vanessaresearch.com
www.vanessaresearch.com
Vanessa Research (VR)—a biomedical company committed to improving patient quality of life by developing innovative products and solutions in areas of unmet medical needs—has acquired Hungaro-Gal, a Hungarian pharmaceutical contract manufacturing company. Per the acquisition, Vanessa Research Holdings Inc. (VRI) will continue Hungaro-Gal’s contract manufacturing services and intends to maintain and grow their existing customer base. Additionally, VRI plans to utilize their facilities to produce Shylicine®, the company’s investigational oral solution developed to treat the rare and lethal microvillus inclusion disease.
Operating out of certified facilities in Kaposvár, Hungary—a small town with a noteworthy, centuries-old tradition in pharmacy—Hungaro-Gal services a growing market need for generic drug substances and fixed dose combinations. For decades, Hungaro-Gal has been the regional center of the Hungarian pharmacy distribution network and small-scale pharmaceutical manufacturing; and for the past two centuries, Hungary has been the most important supplier of medicine for Eastern Europe. Due to Hungary’s well-qualified and cost-effective labor force, outstanding logistical network, and easily accessible location, the country has become a hub for manufacturing and R&D activities for drug and biotech companies.
Bence Krümmer, Managing Director, commented that: “For the past two decades, Hungaro-Gal has provided full-scale manufacturing services that are renowned for their quality and agility and trusted by their partners. It is Vanessa Research’s firm intention to build upon existing customer relationships, keep them and service them, just as Hungaro-Gal has for the last 20 years. Vanessa Research is honored to continue producing the highest quality pharmaceutical and consumer health products and serving pharmacy partners.”
With a continually expanding pipeline as well as offices in Győr and Budapest, Hungary, VRI’s acquisition of Hungaro-Gal signifies an expansion of their existing footprint in Hungary as well as their entrance into the pharmaceutical contract manufacturing business. This transaction also represents a critical step towards VRI’s mission to “give hope where none existed.”
About Vanessa Research
Vanessa Research, Inc. (VRI) is a biomedical company founded on the desire to “give hope where none existed” by making an impact on healthcare markets that are often overlooked. VRI’s continually expanding pipeline of innovative medicine, medical devices, and consumer and public health products reflects the company’s inventive nature and commitment to delivering solutions that increase access to quality care. The company’s flagship products, Shylicine® and Hunazine® are the first-ever drug developed to treat the rare, lethal microvillus inclusion disease and a treatment designed to neutralize the cholera toxin, respectively.
VRI is based in Hamden, Connecticut, with offices in Farmington, Connecticut; London, England; Budapest, Hungary; and Navarre, Spain.
Contact Information
Christina McGrath
Director of Marketing and Communications
christina.mcgrath@vanessaresearch.com
Study will evaluate safety and efficacy of Shylicine®, the first-ever investigational treatment developed for microvillus inclusion disease
Vanessa Research, Inc. (VRI) — a biomedical company founded on the desire to “give hope where none existed” by making an impact on healthcare markets that are often overlooked — has been granted regulatory approval by the Turkish Ministry of Health to conduct a Phase II Clinical Trial for Shylicine®, an investigational oral solution developed to treat the rare and lethal microvillus inclusion disease.
About the Disease
Microvillus inclusion disease (MVID) is an exceedingly rare genetic gastrointestinal disorder that afflicts infants with diarrhea so severe that malnutrition and dehydration are inevitable, and often lethal, if the condition is not appropriately treated.
The current standard of care for MVID is total parenteral nutrition, in which fluids and nutrients are delivered intravenously for up to 24 hours each day. This can often result in infection, damage to the veins, and liver failure. Patients may ultimately seek a small bowel and/or liver transplantation; a process which comes with many challenges and complications of its own.
Vice President of Research and Development, Dmitry Kravtsov, M.D. identified the role that intestinal cell immaturity plays in the severity of diarrhea while working as a research scientist at the Yale School of Medicine. His work suggested that the failure of the MVID intestine may not be permanent, and that the immature cells of the gut could be restored with appropriate intervention – allowing a patient’s intestine to absorb necessary hydration and nutrition. These findings have the potential for broader applications in the field of secretory diarrhea: currently, Vanessa Research is in the preclinical development stage for Hunazine®, a treatment designed to neutralize the cholera toxin in affected patients.
About the Trial
Shylicine® is intended to serve as an oral treatment that could potentially eliminate patients’ dependence on total parenteral nutrition. The twelve-week Shylicine® trial will assess the safety and efficacy of the investigational treatment. The primary endpoint is reduction in the volume of diarrhea and frequency of stool, as well as a decrease in the duration of feeding via total parenteral nutrition.
In addition to receipt of approval to begin the trial, Vanessa Research has been granted a drug import permit, which will allow the U.S.-based company to deliver Shylicine® to the trial site, the Ankara University School of Medicine.
“Authorization by the Turkish Ministry of Health and the support of the Ankara University School of Medicine are critical, given that a relatively high volume of reported MVID cases are located in the country,” explains Norman Gray, CEO of Vanessa Research. “This is a critical step towards addressing the unmet needs of this rare disease community.”
About Vanessa Research
Vanessa Research, Inc. (VRI) is a biomedical company founded on the desire to “give hope where none existed” by making an impact on healthcare markets that are often overlooked. VRI’s continually-expanding pipeline of innovative medicine, medical devices, and consumer and public health products reflects the company’s inventive nature and commitment to delivering solutions that increase access to quality care. The company’s flagship products, Shylicine® and Hunazine® are the first-ever drug developed to treat the rare, lethal microvillus inclusion disease and a treatment designed to neutralize the cholera toxin, respectively.
VRI is based in Hamden, Connecticut, with offices in Farmington, Connecticut; London, England; Budapest, Hungary; and Navarre, Spain.
Contact Information
Contact Information
P.O. Box 185755
Hamden, Conn. 06518 USA
+1 203 836 8424
Contact Us
DISCLOSURE NOTICE
The information contained in this release is as of June 4, 2019. VRI assumes no obligation to update forward‐looking statements contained in this release as the result of new information or future events or developments.
This release contains forward‐looking information about VRI’s Shylicine®, treatment candidate for the indication of secretory diarrhea caused by microvillus inclusion disease, including its potential benefits, and VRI’s plans to initiate Phase 2 trials in the coming months, that involves substantial risks and uncertainties which could cause actual results to differ materially from those expressed or implied by such statements. Risks and uncertainties include, among other things, the uncertainties inherent in research and development, including the ability to meet anticipated clinical trial completion dates and regulatory submission dates, as well as the possibility of unfavorable clinical trial results, including unfavorable new clinical data or additional analyses of existing clinical data; the risk that clinical trial data are subject to differing interpretations, and, even when we view data as sufficient to support the safety and/or effectiveness of a product candidate, regulatory authorities may not share our views and may require additional data or may deny approval altogether; whether regulatory authorities will be satisfied with the design of and results from our clinical studies; whether and when any biologics license applications may be filed in any jurisdictions for Shylicine® for any indications; whether and when any such applications may be approved by regulatory authorities, which will depend on the assessment by such regulatory authorities of the benefit-risk profile suggested by the totality of the efficacy and safety information submitted and, if approved, whether Shylicine® will be commercially successful; decisions by regulatory authorities regarding labeling and other matters that could affect the availability or commercial potential of Shylicine®; uncertainties regarding the ability to obtain recommendations from technical committees and other public health authorities as well as uncertainties regarding the commercial impact of any such recommendations; and competitive developments.
Vanessa Research (VR) – a Connecticut-based biomedical
company discovering, developing, and commercializing innovative products in
areas of unmet medical needs – announced the appointment of A. Donald Janezic,
CPA, CGMA as Chief Financial Officer, effective June 1, 2019. Janezic joins as
a key member of the expanding company’s executive leadership team, and as a visionary
strategist expected to drive the international success of VR’s brand portfolio.
Janezic is a graduate of Bentley University (BSA, 1978) and
the University of New Haven (MBA, 1983). He is a Certified Public
Accountant and an active member of the Connecticut Society of CPAs (CTCPA)
along with a myriad of other professional organizations. Previously, he served
as a senior accountant for KPMG, and a law firm administrator for MCRP, both in
Hartford CT. He was also CFO for two manufacturing companies, most recently
Bigelow Tea of Fairfield CT where he retired after 30 years of service.
Along with his influential management position, Janezic is a
member of the Vanessa Board of Directors and will lead all financial activity
of VR’s international operations as CFO.
Commenting on his appointment, Janezic stated: “Joining the executive team at Vanessa Research is both exciting and challenging as we take the company from unique product research and development to actual production and distribution on an international scale. The acquisition of the GMP-certified pharmaceutical manufacturing facilities in Hungary will further permit Vanessa Research to fulfill its mission to ‘give hope where none existed’ – our promise to the world.”
About Vanessa Research
Vanessa Research (VR) is a biomedical company founded on the desire to “give
hope where none existed” by making an impact on healthcare markets that are
often overlooked. VR’s continually-expanding pipeline of innovative
pharmaceuticals, medical devices, and consumer and public health products
reflects the company’s inventive nature and commitment to delivering solutions
that increase access to quality care. The company’s flagship products,
Shylicine® and Hunazine® are the first-ever drug developed to treat the rare,
lethal microvillus inclusion
disease and a treatment designed to neutralize the cholera toxin,
respectively.
VR is based in Hamden,
Connecticut, with offices in Farmington, Connecticut; London, England;
Budapest, Győr, and Kaposvár, Hungary; and Navarre, Spain.
Contact Information
Christina McGrath
Director of Marketing and Communications
christina.mcgrath@vanessaresearch.com
Vanessa Research brings awareness of Microvillus Inclusion Disease to healthcare professionals worldwide
HAMDEN, CT, May 8, 2019 — Vanessa Research, Inc. (VRI) — a biomedical company transforming the healthcare landscape by developing and commercializing innovative products in areas of unmet medical needs — participated in an interview with Health Professional Radio (HPR), a health-dedicated media channel that targets medical professionals primarily in hospital-based environments. Dr. Dmitry Kravtsov, M.D, Vice President of Research and Development at VRI, joined HPR to provide expert knowledge on the very rare, genetic microvillus inclusion disease (MVID). The interview is available at healthprofessionalradio.com.au/microvillus-inclusion-disease-mvid/.
During the interview, Dr. Kravtsov addressed treatment options for MVID, a pediatric disease that afflicts infants with persistent and severe diarrhea resulting in malnutrition, stunted growth, and, in most cases, significantly shortened lifespans. He provided insight on how his strong interest for biomedical research caused him to lead the development team at VRI in creating a patented, non-invasive treatment for MVID called Shylicine®. As part of the program, Dr. Kravtsov also discussed VRI’s white paper, “Addressing the Microvillus Inclusion Disease Knowledge Gap – A Comprehensive Case Analysis,” which is the first and only exhaustive analysis that summarizes data trends across all known literature on MVID.
In response to a question posed about the MVID knowledge gap, Dr. Kravtsov stated: “The current knowledge gap is that yes, the disease is known however it’s only known to…a handful of physicians and a handful of pediatricians in the world. [Most healthcare professionals] are completely unaware of the disease and worse, since this is a rare disease, there’s only a few hundred of cases that have been reported…it’s a huge gap in the understanding between the public and the general medical community.”
By addressing and raising awareness of this rare disease, VRI furthers its ongoing mission to serve overlooked healthcare markets. VRI plans to continue its global spread of information on this lethal disease, fulfilling the company’s mission to “give hope where none existed.’’
About Vanessa Research
Vanessa Research, Inc. (VRI) is a biomedical company founded on the desire to “give hope where none existed” by making an impact on healthcare markets that are often overlooked. VRI’s continually-expanding pipeline of innovative pharmaceuticals, medical devices, and consumer and public health products reflects the company’s inventive nature and commitment to delivering solutions that increase access to quality care. The company’s flagship product, ShylicineTM, is the first-ever drug developed to treat the rare, lethal microvillus inclusion disease.
VRI is based in Hamden, Connecticut, with offices in Farmington, Connecticut; London, England; Budapest, Hungary; and Navarre, Spain.
Contact Information
Christina McGrath
Marketing and Communication Specialist
Vanessa Research, Inc.
925 Sherman Avenue,
Hamden, CT, 06514 USA
christina.mcgrath@vanessaresearch.com
www.vanessaresearch.com
Vanessa Research brings cutting-edge science to the world-renowned pediatric congress
Vanessa Research, Inc. (VRI) — a biomedical company that is transforming the healthcare landscape through the development of innovative products and solutions in areas of unmet medical needs — announced it will be a silver sponsor of the 17th Congress of the European Society for Developmental, Perinatal and Paediatric Pharmacology (ESDPPP). The growing start-up will provide critical contributions to the congress as a sponsor and speaker along with other distinguished, multinational corporations. The event will be held May 28-30, 2019 at the Congress Center Basel—the largest conference center in Switzerland.
The primary goal of ESDPPP is to promote research in the field of pediatric pharmacology and to offer a forum for exchange between pharmacologists and clinical physicians about more adequate drug administration. The society’s biennial congress serves as a platform for physicians, pharmacists, nurses, and translational and clinical scientists working in academia, regulatory agencies, and pharmaceutical companies to coalesce on discussions relevant to the science and development of pediatric medicines. ESDPPP is accredited by three societies and works closely with European Medicines Agency (EMA), International Union of Basic and Clinical Pharmacology (IUPHAR), and the World Health Organization (WHO).
On Wednesday, May 29 the Vice President of Research and Development at VRI, Dr. Dmitry Kravtsov, will be presenting on the topic of drug development study design for rare diseases. Kravtsov’s presentation will focus on treatment for the rare, genetic microvillus inclusion disease (MVID). MVID is a pediatric disease for which VRI has been relentlessly working to develop a treatment. Infants with MVID suffer from persistent and severe diarrhea that results in malnutrition, stunted growth, and, in most cases, significantly shortened lifespans. By addressing and raising awareness of this rare disease, VRI promotes its ongoing mission to serve overlooked healthcare markets.
Commenting on his upcoming presentation at the ESDPPP Congress, Kravtsov stated: “This event is another opportunity for VRI to provide a comprehensive analysis and understanding of MVID so that the best treatment options may be developed. Our company plans to continue its global spread of information on this lethal disease, fulfilling our mission to ‘give hope where none existed.’’”
About Vanessa Research
Vanessa Research, Inc. (VRI) is a biomedical company founded on the desire to “give hope where none existed” by making an impact on healthcare markets that are often overlooked. VRI’s continually-expanding pipeline of innovative pharmaceuticals, medical devices, and consumer and public health products reflects the company’s inventive nature and commitment to delivering solutions that increase access to quality care. The company’s flagship product, ShylicineTM, is the first-ever drug developed to treat the rare, lethal microvillus inclusion disease.
VRI is based in Hamden, Connecticut, with offices in Farmington, Connecticut; London, England; Budapest, Hungary; and Navarre, Spain.
Contact Information
Christina McGrath
Marketing and Communication Specialist
Vanessa Research, Inc.
925 Sherman Avenue,
Hamden, CT, 06514 USA
christina.mcgrath@vanessaresearch.com
www.vanessaresearch.com
– A rare, fatal intestinal disease that victimizes infants resulting in poor quality of life and a short lifespan if not diagnosed and treated-
HAMDEN, Conn., February 28, 2019 — Vanessa Research, Inc. (VRI) – a Connecticut-based biomedical company discovering, developing, and commercializing innovative products in areas of unmet medical needs – announced today the publication of a white paper, the first and only comprehensive case analysis that focuses on all known case studies published globally on microvillus inclusion disease (MVID). MVID is an exceedingly rare genetic gastrointestinal disorder that afflicts infants with diarrhea so severe that malnutrition and dehydration are inevitable, and often lethal if the condition is not appropriately diagnosed and treated.
The white paper, Addressing the Microvillus Inclusion Disease Knowledge Gap – A Comprehensive Case Analysis, is the first and only comprehensive analysis that summarizes data trends across all known literature on MVID. The authors aggregated data related to patient location, gender, disease onset, and various treatment strategies.
Dr. Dmitry Kravtsov, M.D., one of the authors and Vice President of Research & Development at VRI, stated that: “Given the rarity of microvillus inclusion disease, it can be difficult for doctors and caregivers to find information and research related to the condition. Vanessa Research is incredibly excited to publish this paper as part of its ongoing mission to serve as a premiere educational resource on MVID and to raise awareness of this devastating disorder.”
The paper underscores the fact that the treatment methods currently considered to be the most effective for management of MVID — total parenteral nutrition (TPN), in which fluids and nutrients are delivered via IV directly into the bloodstream of the patient, and eventually a small bowel transplantation — are associated with not only a poor quality of life for patients, but also can lead to serious and potentially lethal complications.
The report concludes that the accelerated development of new treatment options for long-term management of MVID is needed given the poor prognosis and quality of life associated with the standard methods of care.
While the precise prevalence of MVID is unknown, only 356 cases have been reported in the literature since the first case study in 1978. Cases remained relatively high from 1991 – 2017 with a notable spike of 147% cases. The incredibly small population of patients presents challenges regarding the breadth and availability of scientific and medical information pertaining to the disease.
“VRI plans to continue increasing awareness and reliable information on MVID globally, fulfilling the company’s mission to ‘give hope where none existed’ by focusing on diseases that are overlooked due to their rarity,” said Dr. Kravtsov. “This paper is one of the many first steps by our company to providing an understanding of this fatal disease so that the best treatments may be developed.”
Addressing the Microvillus Inclusion Disease Knowledge Gap – A Comprehensive Case Analysis can be downloaded now at mvid.vanessaresearch.com/case-analysis.
January 28, 2019 | Hamden, CT – Vanessa Research, Inc. (VRI) is pleased to announce that its MIDA™ (Microvillus Inclusion Disease Assay) project, under the direction of Dr. Ludmila Kvochina, has been accepted into the 2019 cohort of the highly selective Accelerator for Biosciences in Connecticut (ABCT).
MIDA™ is the first-ever dipstick test developed to screen for the incredibly rare and lethal microvillus inclusion disease.
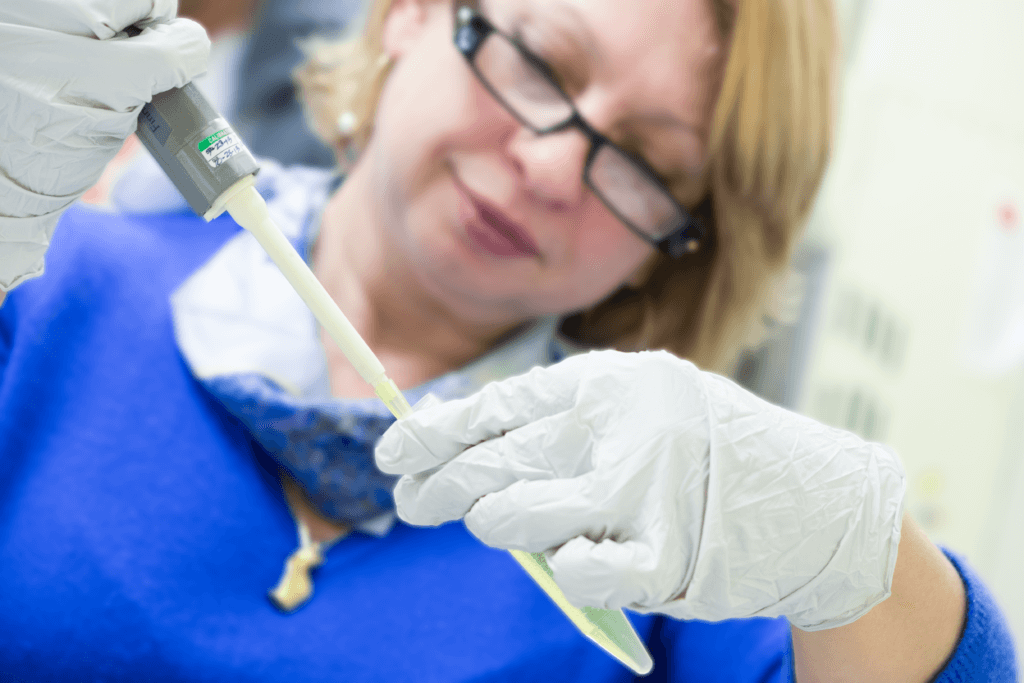
Dr. Ludmila I. Kvochina, Director of Research & Development Laboratory at Vanessa Research, has been working to develop a high throughput screening test for detection of microvillus inclusion disease in infants presenting with diarrhea.
ABCT’s competitive-entry six-month-long program helps emerging bioscience ventures grow by providing entrepreneurship education and business networking to access global funders and prospective team members.
ABCT’s goal is to support the development of Connecticut’s hub of bioscience innovation and commercialization.
The MIDA™ project was selected following an extensive screening process that involved evaluation by expert scientists, active investors, and business strategists based on the strength of the team, uniqueness of insight, and capacity to revolutionize the life sciences industry.
Microvillus Inclusion Disease (MVID) is a rare intestinal disorder presenting early in affected infants’ lives, with patients experiencing severe diarrhea that renders them unable to absorb fluids, nutrients, or electrolytes – meaning that malnutrition and dehydration are inevitable and often lethal if the condition is not appropriately recognized and treated. Many children affected by MVID may not live to see their third birthday.
MVID is often misdiagnosed – or not diagnosed at all – due to a general lack of awareness of the disease. MIDA™ intends to bring MVID into the standard differential diagnosis for infants presenting with diarrhea; potentially aiding in raising general awareness of the disease among physicians and thus, decreasing rates of misdiagnosis, delayed diagnosis, or missed diagnosis of this rare genetic disorder.
Dr. Kvochina, Director of the Research & Development Laboratory at VRI, commented that: “With the supportive community and entrepreneurial expertise that ABCT fosters, MIDA™ will one day allow any hospital or healthcare professional – despite location, equipment, or specialty – to easily screen for this incredibly rare and under-recognized disease. The ability to detect microvillus inclusion disease is a crucial step towards appropriate treatment, and therefore survival, of impacted children.”
MIDA™ is being developed to accompany VRI’s company mission to “give hope where none existed” by filling the unmet need for a reliable and simple means to screen for microvillus inclusion disease. MIDA™ joins VRI’s expanding portfolio of products addressing diarrheal diseases, including Shylicine™, the first drug designed to treat MVID; and Hunazine™, a treatment that is being developed to neutralize the Cholera toxin in affected patients.
While MIDA™ is new to the Accelerator for Biosciences in Connecticut program, Vanessa Research is not. The company’s SOLaware™ Display – a device that could be found informing beach and park visitors of local UV conditions and sun safety tips across CT last summer – was a participant in the ABCT’s inaugural 2018 cohort, and among the program’s first projects to generate customer revenue.

About Vanessa Research
Vanessa Research is based in Hamden, Connecticut, with offices in Farmington, Connecticut; London, England; Budapest, Hungary; and Navarre, Spain. VRI was founded on the desire to build a 21st Century, sustainable, innovative, entrepreneurial organization by bringing together a collection of diverse scientists, engineers, business professionals, and academics to create solutions in medicine. The company’s mission is to “give hope where none existed” by delivering healthcare products that improve and increase access to quality care while decreasing costs.
Contact Information
Christina McGrath
Marketing and Communication Specialist
christina.mcgrath@vanessaresearch.com
www.vanessaresearch.com


